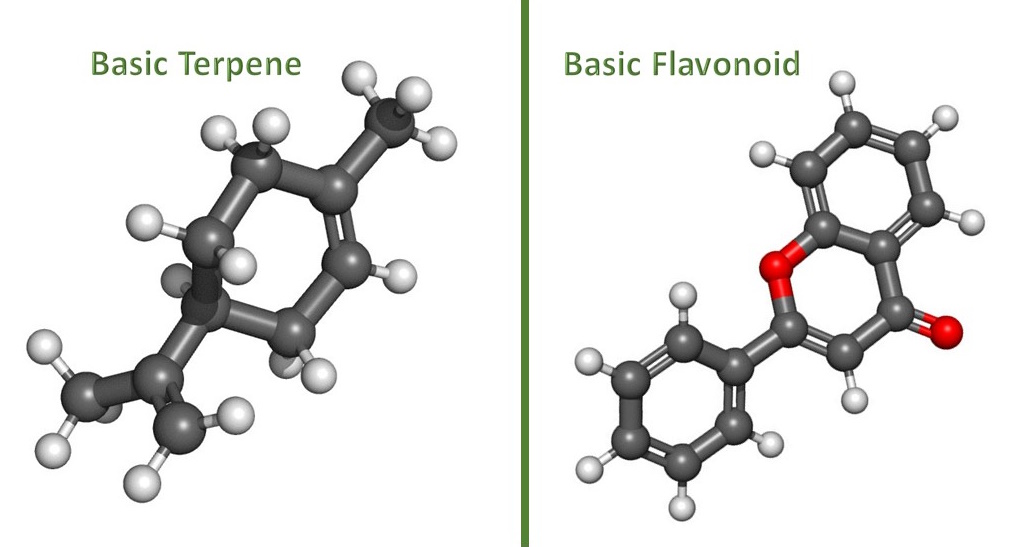
The following are 4 primary differences between terpenes and flavonoids. This information is derived from Edition 2 of The Big Book of Terps.
MOLECULAR WEIGHT
Terpenes: the average molecular weight of terpenes and terpenoids that are common to cannabis is 177.3 g/mol.
Flavonoids: the average molecular weight of flavonoids that are common to cannabis is 378.4 g/mol.
This means that flavonoids are much heavier, and therefore far less likely to volatilize than terpenes and terpenoids. The flavonoid molecule is more stable than the small and volatile terpene molecule.
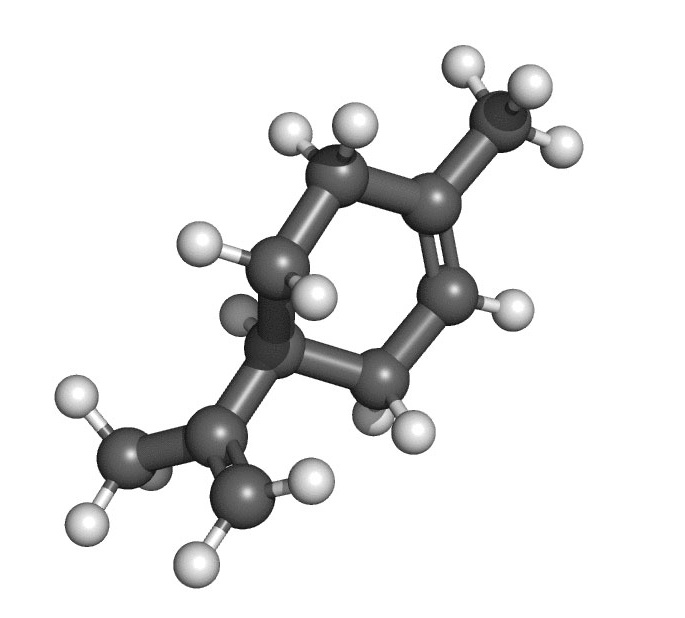
MOLECULAR STRUCTURE
Terpenes: Terpenes are formed in plants by combining two acetic acid molecules to form isoprene (also referred to as 2-methylbutane) via isopentenyl pyrophosphate as a precursor. The mother hydrocarbon, isoprene, has a formula of C5H8, which denotes the fact that isoprene has five carbon atoms bound to eight hydrogen atoms. To form a terpene, two or more of these units or molecules of isoprene must combine. This means that most terpenes start with a C10H16 configuration.
Flavonoids: Plants produce flavonoids initially in the phenylpropanoid metabolic pathway, where an amino acid creates several substances leading to the development of a compound containing two benzene rings, referred to as Ring A and Ring B. These two rings are joined in the center by a 3-carbon ring of pyran containing an oxygen atom, called Ring C. This forms the classic fifteen carbon skeleton structure of flavonoids, notated as C6C3C6.
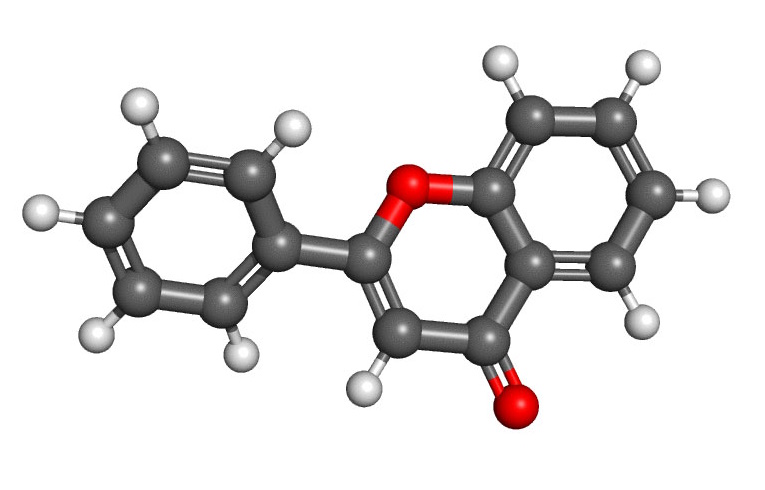
MOLECULAR CONTENT
Terpenes often occur as smaller, volatile molecules prone to molecular rearrangement, which means that they often occur as different isomers, which are the same molecule but with differences in structure or position.
Flavonoids, on the other hand, don’t often occur as isomers – they are much larger and more stable molecules and not so prone to rearrangement. Instead, flavonoid molecules can take on additional functional groups, for instance via glycosylation (the addition of a sugar atom or moeity), or through the addition of sulfates (addition of a sulfur atom), hydrates (addition of a water atom), and other modifications that can change the properties of the molecule.
HUMAN PERCEPTION
In general, humans mostly perceive terpenes as smells and flavors, while we perceive flavonoids primarily as colors.